Dear All (and with thanks to Patricia Bradford for leading on this newsletter),
Did you know you can access raw datasets of antimicrobial susceptibility surveillance data that have been generated by the pharmaceutical industry? Indeed such datasets exist and we’d like to ensure everyone is aware of their availability and potential!
Background: The 20 Oct 2023 newsletter discussed Vivli, a nonprofit entity dedicated to advancing human health through data sharing. Vivli supports a clinical data sharing platform that was created in 2013 from a project by the Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard.
The AMR focus: Vivli seeks to enable innovative re-use of surveillance data shared by GSK, Johnson & Johnson, Merck, Pfizer, Shionogi, Paratek, Venatorx, and Venus Remedies. Vivli was funded in June 2022 by Wellcome to launch the AMR Register (amr.vivli.org). The AMR Register provides access to high-quality antimicrobial susceptibility surveillance data (MIC by organism) generated by the pharmaceutical industry.
- Surveillance programs conducted by industry are mandatory and comply with regulatory agency requirements for commercial approval and post-approval commitments for new antimicrobial agents.
- By sharing their data via the AMR Register, pharmaceutical companies thereby uphold their agreements with regards to data transparency.
- The AMR register currently has nine member companies:

What’s in the data?
- The data hosted in the Register consists of raw minimum inhibitory concentration (MIC) data from pathogen susceptibility studies.
- All MICs were generated using reference methods.
- There are currently data on more than 1,000,000 isolates from 89 countries!
- Datasets include aerobic bacteria, anaerobic bacteria, fungi, and mycobacteria:

How can you access these data?
- Review the details of the available surveillance programs
- Log onto the register interface at https://searchamr.vivli.org/
- Select organism, antimicrobial or demographic of interest
- Search will return a list of datasets that contain information in your search
- Submit a request for datasets you wish to obtain (entire dataset, not a cut containing your search criteria)
- After a short review period, datasets will be provided for download in an Excel format. These contain MIC by pathogen and some basic patient demographics.
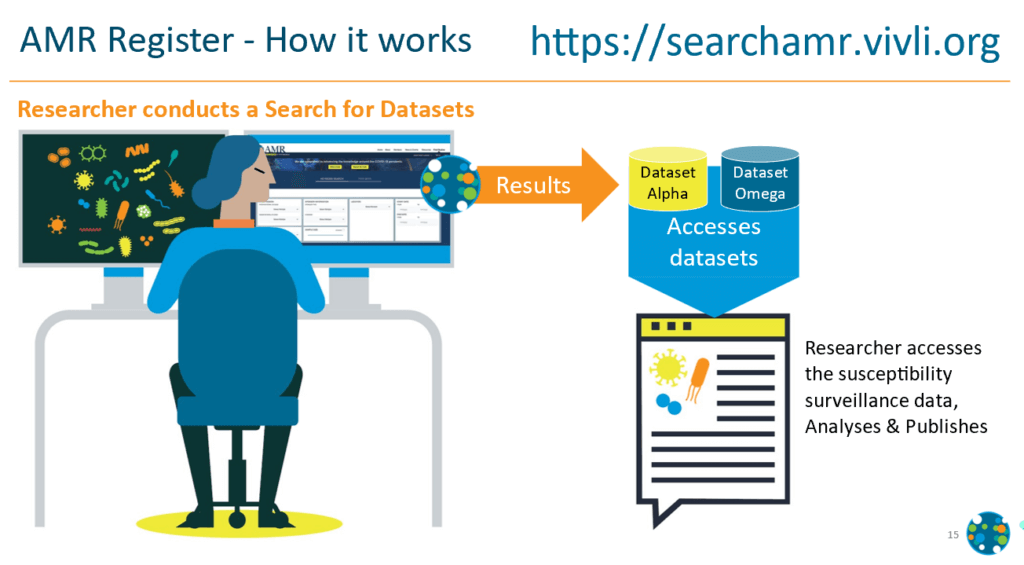
What can you do with that data?
It’s up to you! Resistance trends over time? Predict future trends? Guide local or regional stewardship? Be creative! Results from a data challenge conducted in 2023 should give you some ideas:
- The team that won the 2023 Grand Prize was from Kenya and built an AI tool that predict local susceptibility of a pathogen by using the limited data from Africa and comparing it to data from other sources.
- Other prize winners for innovation and impact were projects on
- The potential and limitations of combining industry datasets with data from WHO’s GLASS to fill in global AMR surveillance gaps
- Global geographic patterns and trends of WHO priority pathogens and AWaRe antibiotic resistances among children
- Are antibiotic breakpoints globally consistent, and does it matter if not? (a comparison of CLSI and EUCAST breakpoints)
- Exploration of the association between regional resistance patterns and global climactic variables, such as humidity, temperature and precipitation.
- Prize winners and their creative solutions can be found on the AMR register at https://amr.vivli.org/data-challenge/finalist-and-award-winning-solutions/. In addition, several projects have been published in Wellcome Open Research.
- The 2024 data challenge is in progress, so look for results to be announced later this year.
Questions? Contact Patricia Bradford pbradford@antimicrobialdev.com or through the AMR register contact page https://amr.vivli.org/contact/
There is a goldmine of data available for your use — all you have to do is ask!
With all best wishes, Patricia & John
Patricia Bradford, PhD | Antimicrobial Development Specialists, LLC | pbradford@antimicrobialdev.com. All opinions are my own.
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
- ENABLE-2 has continuously open calls for both its Hit-to-Lead program as well as its Hit Identification/Validation incubator. Applicants must be academics and non-profits in Europe due to restrictions from the funders. Applications are evaluated in cycles … see the website for details on current timing for reviews.
- CARB-X has open calls at intervals that span four areas: (i) Therapeutics for Gram-Negatives, (ii) Prevention for Invasive Disease, (iii) Diagnostics for Neonatal Sepsis, and (iv) Proof-Of-Concept for Diagnosing Lower-Respiratory-Tract Infections. See this 6 Mar 2024 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. There are multiple opportunities to submit — see the CARB-X webpage for details.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- 25-26 February 2025 (Basel, Switzerland): The 9th AMR Conference 2025. Go here to register!
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
- (no date as yet) 2025 ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here to see details of the outstanding 2024 meeting!
- 19-22 Oct 2025 (Georgia, USA): IDWeek 2025, the annual meeting of the Infectious Diseases Society of America. Details pending; go here for the general meeting website.
Upcoming meetings of interest to the AMR community:
- 22-24 Oct 2024 (Belgrade, Serbia): Ecraid/ESCMID postgraduate course “Better methods for clinical studies in infectious diseases and clinical microbiology”. Go here to register by 29 Sep 2024.
- 4-5 Dec 2024 (in person, Washington, DC): “Fungal Dx 2024: Fungal Diagnostics in Clinical Practice” is a 2-day in-person workshop organized by ISHAM‘s Fungal Diagnostics Working Group. The program and registration links are available at https://fungaldx.com/; the agenda is comprehensive and features an all-star global list of speakers.
- [NEW — very important meeting] 28-29 Jan 2025 (online and in-person, Washington, DC): PACCARB (US Presidential Advisory Council on Combatting Antimicrobial Resistant Bacteria): This particular meeting of PACCARB is unusually important as it will seek (i) public input into NAP for CARB 2025-2030 and (ii) work to sustain the momentum regarding the commitments at the High-Level Meeting on AMR at the 2024 UN General Assembly (UNGA HLM AMR). Go to http://hhs.gov/paccarb for details and to register.

- 4-5 Feb 2025 (online, 1-5p GMT timing on both days): Antimicrobial Chemotherapy Conference by GARDP and BSAC in collaboration with CEPID-ARIES and Fiocruz. Now in its 6th year, the free program offers a good review of antimicrobial R&D, ranging from drug discovery to preclinical and clinical activities. Go here to register; the abstract deadline is 15 Nov 2024.
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.
- 19-22 Oct 2025 (Georgia, USA): IDWeek 2025, the annual meeting of the Infectious Diseases Society of America. See Recurring Meetings list, above.
- OpenWHO: “Antimicrobial Resistance in the environment: key concepts and interventions.” Per the webpage for the course, it will teach you “…why addressing AMR in the environment is essential and gain insights into how action can be taken to prevent and control AMR in the environment at the national level.” This course builds on WHO’s 2024 Guidance on wastewater and solid waste management for manufacturing of antibiotics. For further reading, see also the 25 Sep 2023 newsletter entitled “Manufacturing underpins both access and stewardship: Cefiderocol as a case study” and the 28 Jan 2024 newsletter entitled “EMA Concept Paper: Guidance on manufacturing of phage products”.