Dear All,
Just published in AAC is WHO’s updated review of the global preclinical pipeline. Here are the links you need to follow today’s discussion:
- The new WHO review: Gigante et al. “Multi-year analysis of the global preclinical antibacterial pipeline: trends and gaps”, https://doi.org/10.1128/aac.00535-24, AAC 2024.
- An excellent AAC podcast (overview webpage; YouTube video) discussing the WHO review.
- In this broad-ranging podcast, Cesar Arias (AAC Editor-in-Chief and a co-author on the paper) and Valeria Gigante (lead author and Team Lead in WHO’s AMR Division) discuss WHO’s priority pathogen lists, the review methodology for pipeline review, and WHO’s findings over time regarding the dynamics of both clinical and preclinical antibacterial research.
- Relevant prior papers and newsletters
- All prior reviews by WHO: https://amr.solutions/pathogens-and-pipelines/#pipeline-reviews. The most relevant is the WHO 2023/24 clinical pipeline review with data cut-off of 31 Dec 2023, published in 2024: My newsletter is here and the report is here.
- The 5-part series on the challenge(s) of AI-based discovery that began 21 Feb 2020 with a newsletter entitled “Chemicals Vs. Drugs (Part 1): The End Of Bacitracin / The Buzz Around Halicin.”
- The 9 April 2024 newsletter on a traditional discovery program entitled “48,015 → 0: Antibacterial Discovery Is Hard. Really, Really Hard.”
- The 8 Feb 2024 newsletter by the AMR Industry Alliance on the declining pool of AMR-focused researchers: “Leaving The Lab: The Decline In AMR R&D Professionals.”
- The 30 June 2020 newsletter discussing the findings by Dheman et al. in their review of FDA’s 40-year perspective on antibacterial development: the number of programs is small and the average during of clinical development is approaching 9 years.
- An almost simultaneous review (accepted 17 June 2024) from the Centre for Superbug Solutions in Queensland: Butler MS et al. “A Review of Antibacterial Candidates with New Modes of Action”, https://doi.org/10.1021/acsinfecdis.4c00218, ACS Infectious Diseases, 2024.
As you may have already guessed from the title of the newsletter (Needles? Haystacks? Hmm!), today’s newsletter continues our lament regarding the difficulty with finding antimicrobials that are actually drugs and not just poisons.
The paper by Gigante et al. (and full disclosure: I am also part of the “et al.”) is a summation of an effort by WHO over the past 4 years to review the preclinical pipeline. The frustrating messages from this paper can be summarized as:
- The pool of projects remains small: At any given time, there are 200-250 projects underway
- The work is being done 120-150 development groups, mostly at small companies by ~3,000 researchers (the AMR Industry Alliance report discussed on 8 Feb 2024).
- There is a lot of churn: only ~19% of the groups have been across all 4 years.
- In an encouraging sign, the number of programs in IND-enabling studies (that is, late-preclinical studies, verging on starting human Phase 1 studies) was up 25% in 2023.
- Direct-acting small molecules and peptides are steady at ~60% of programs
- About 30% of programs are narrow-spectrum (mostly M. tuberculosis or P. aeruginosa)
- Phage are steady at ~15% of programs
- But, the big negative (pardon the pun) is that number of programs focused on priority Gram-negative pathogens is in the range of 50-100. When you consider the tiny % of the programs that will progress to Phase 1 and the even smaller % that will progress to approval, this pool of candidates is scarily small:
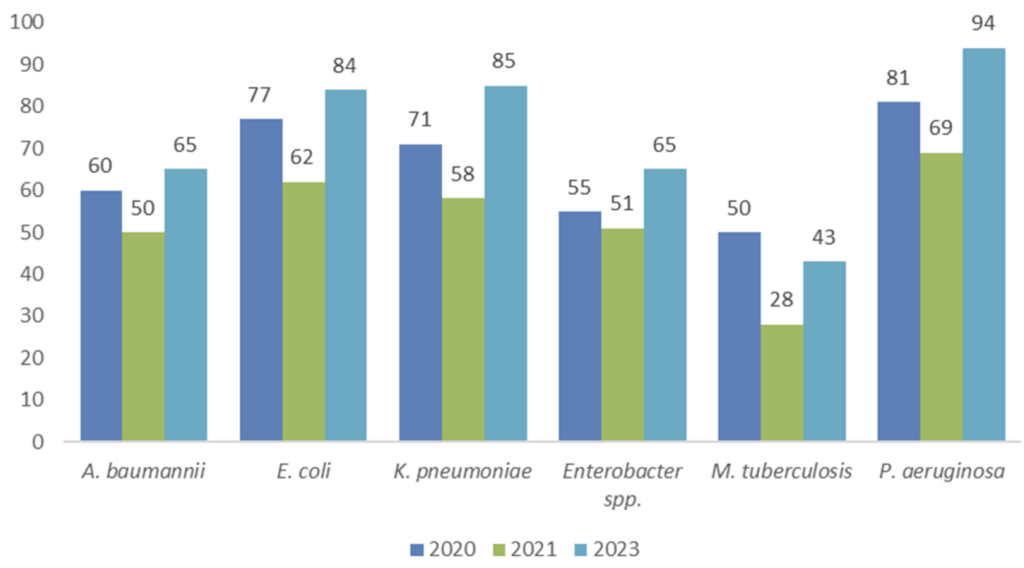
Above: Figure 8 from Gigante et al. Shown by year is the number of programs targeting each pathogen. Some programs will have been counted more than once.
The prior supporting papers are hopefully well known to you from prior newsletters, so I’ll not recite at length — the recurring message about the cause(s) of the thin pipeline and the slow place of antibacterial R&D is that it’s easy to kill bacteria but it’s hard to do that while also being drug-like and at least reasonably non-toxic (for a punchy visual summary of this message, see the 25 Jan 2020 newsletter).
—
Moving on to the final paper, we have an impressively detailed review of novel antibacterial by the University of Queensland’s Centre for Superbugs Solutions that has published just a few weeks ago. These authors (Butler et al.) are encouraged by the diversity of molecules they have identified and conclude that intriguing programs are stalling due to insufficient funding. That may well be true, but I think the Gigante review across the entire industry suggests that typical program-limiting issues (toxicity, formulation) are common and substantial.
Whether they are right of wrong, I do commend Butler et al. for their excellent summary. And in particular, I would draw your attention to Figure 1 and Table 1 from their paper as these provide a complete tour of all antibacterial mechanisms and classes. Equally helpful are the further mechanistic details (with mechanistic cross-references) in Figures 3 and 7.
—
Grumble, grumble. What’s to be done? What’s to be done differently? The search for new antimicrobial agents really is like searching for a needle in a haystack!
I am encouraged by the level of funding (CARB-X, ENABLE-2, AMR Action Fund, etc.) now in play and will keep my fingers crossed — just 2-3 really novel programs per decade would make a big difference. But it really is a marathon rather than a sprint: we need to sustain the people who do the work over those many years. Did I hear someone say “PASTEUR Act?” Yes, please!
With all best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
- [DEADLINE UPDATE] Vivli has announced the 2024 Vivli AMR Surveillance Data Challenge. This particular challenge is funded by GARDP, Paratek, Pfizer and Vivli and aims to encourage and support the innovative re-use of surveillance data shared by GSK, Johnson & Johnson, Pfizer, Shionogi, Paratek and Venatorx that are now available in the Vivli AMR Register. This is 2nd AMR data challenge from Vivli — see the 20 Oct 2023 newsletter for a discussion of the outcome of this first challenge. This 2nd challenge offers monetary prizes, including travel funding to attend ESCMID Global or ASM Microbe in 2025 (if an abstract is accepted), and a new AMR Student Innovation Award. The deadline for expressions of interest has been extended to 11 Aug 2024. For more details, go here; you might also review the GARDP REVIVE webinar on 23 Jul 2024 that focused on the program.
- Environmental AMR issues, anyone? ICARS has call open through 1 Aug 2024 for “projects in the public health sphere that aim to mitigate the evolution and transmission of resistance in the natural or built environment.” Grants are available of up to $800k for up to 4 years. Go here for details; for questions and submissions, write to RFP_EDAR@icars-global.org. Applicants should also refer to “Mitigating antimicrobial resistance (AMR) using implementation research: a development funder’s approach” from JAC 2023 (https://doi.org/10.1093/jacamr/dlad031).
- [NEW] The GHIT Fund has announced its 21st Request for Proposals for its Hit-to-Lead Platform to support drug discovery and development process to address Malaria, Tuberculosis, Chagas disease, and Visceral leishmaniasis. Go here for the RFP: GHIT-RFP-HTLP-2024-002. The deadline is 30 Aug 2024.
- The AMR Industry Alliance have announced the 2024 edition of their ongoing annual series of stewardship prizes. Applications for innovative approaches to AMR stewardship are sought from public, private or not-for-profit health care organization or institution operating in an LMIC. This year’s deadline is 1 Sep 2024. Go here for details.
- ENABLE-2 has continuously open calls for both its Hit-to-Lead program as well as its Hit Identification/Validation incubator. Applicants must be academics and non-profits in Europe due to restrictions from the funders. Applications are evaluated in cycles … see the website for details on current timing for reviews.
- CARB-X has open calls at intervals that span four areas: (i) Therapeutics for Gram-Negatives, (ii) Prevention for Invasive Disease, (iii) Diagnostics for Neonatal Sepsis, and (iv) Proof-Of-Concept for Diagnosing Lower-Respiratory-Tract Infections. See this 6 Mar 2024 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. There are multiple opportunities to submit — see the CARB-X webpage for details.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here to register!
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. Save the date! More details to come!
- 25-26 February 2025 (Basel, Switzerland): The 9th AMR Conference 2025. Go here to register!
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
Upcoming meetings of interest to the AMR community:
- 22 Aug 2024 (virtual, 11a-12.30p EDT, 5p-630p CEST): GARDP REVIVE Webinar entitled “Exploring non-traditional antimicrobials: Insights from three cases.” Go here for details and to register. If non-traditional approaches interest you, please do be sure to review the challenges that are raised in the papers discussed in the 6 Aug 2019 newsletter entitled “Non-Traditional Antibiotics: A Pipeline Review And An Analysis Of Key Development Challenges.” Developing non-traditional products is MUCH harder than you might expect … it is important to know the issues!
- [NEW – DON’T MISS IT] 28 Aug to 28 Sep (Off-Broadway, New York City, the Alice Griffin Jewel Box Theatre): Lifeline, the musical story of Sir Alexander Fleming’s discovery of penicillin. Previously entitled The Mould that Changed the World, the musical is a two-time Edinburgh Festival Fringe sell-out (2018 and 2022) and has toured to London, Glasgow, Atlanta and Washington DC (2022). This 5-week run in NYC is timed to be in support of the High-Level Meeting on AMR (HLM AMR) during UNGA 2024. Go here for a blurb and here to book your tickets!
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. See Recurring Meetings list, above.
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. See Recurring Meetings list, above.
- 19-27 Oct 2024 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance). Now in its 8th year, Patrice Courvalin directs the program with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications open in March 2024 — go here for more details.
- 4-5 Dec 2024 (in person, Washington, DC): “Fungal Dx 2024: Fungal Diagnostics in Clinical Practice” is a 2-day in-person workshop organized by ISHAM‘s Fungal Diagnostics Working Group. The program and registration links are available at https://fungaldx.com/; the agenda is comprehensive and features an all-star global list of speakers.
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.