Dear All,
WHO have just released a 2024 update to their 2017 (bacterial) Priority Pathogen List (PPL)! Here are the links you need:
- The WHO 2024 PPL.
- WHO’s webpage about the 2024 PPL.
- A PowerPoint (.pptx) deck (and there is also a .pdf version) summarizing the new PPL and all prior PPLs. 22 May 2024 post-newsletter update: I’ve learned of a 2021 PPL from Japan and it is now included in the downloadable documents.
- https://amr.solutions/pathogens-and-pipelines/, the AMR.Solutions webpage listing all prior PPLs (including the 2022 WHO fungal PPL) as well as pipeline reviews to date.
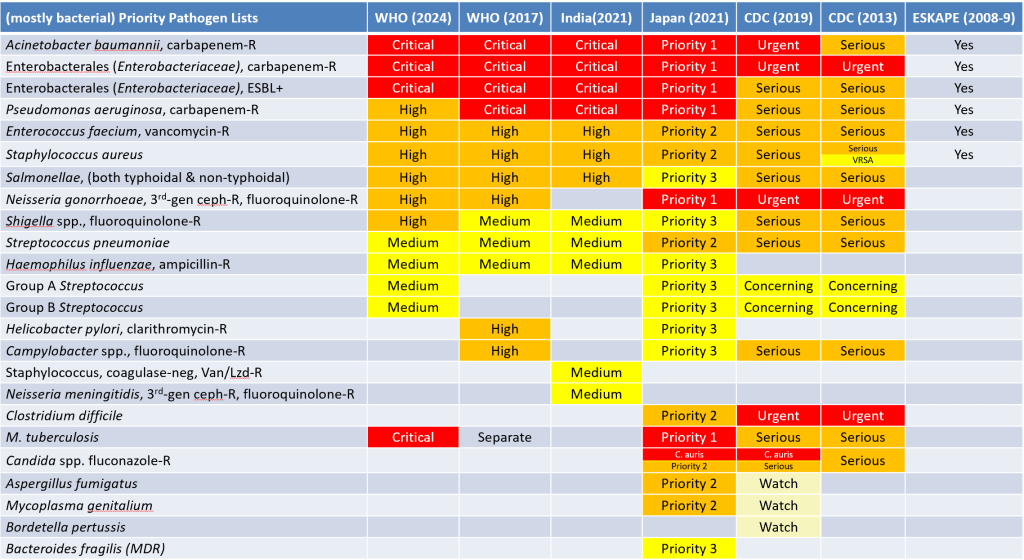
To create the update, WHO have again used MCDA (multi-criteria decision analysis) based on mortality, incidence, non-fatal burden, resistance trends, transmissibility, preventability, treatability, and pipeline quality as ranking factors. Highest weight went to treatability and mortality. Equal but lesser weight went to incidence, non-fatal burden, transmissibility, and preventability.
The changes in the WHO PPL relative to their 2017 PPL and to other PPLs are best considered visually. To get adequate resolution in the chart, I suggest you download the .pptx (or the .pdf) of my cross-tabulation of the PPLs to date.
In brief, here are the key changes relative to WHO’s 2017 PPL:
- Carbapenemase-producing P. aeruginosa moves down from Critical to High.
- This may seem a bit surprising, but the report explains that reduction in rates of resistance and lower transmissibility caused this shift.
- The report also notes that the GBD (global burden of disease) due to P. aeruginosa is significant but at the lower end of the major pathogens (see the 29 Apr 2024 and 20 Jan 2022 newsletters on GBD due to infection and resistance for more details).
- I also note that this shift aligns with CDC’s 2019 ranking of this pathogen as Serious rather than Critical.
- Removed: Clarithromycin-resistant Helicobacter pylori and fluoroquinolone-resistant Campylobacter spp.
- Added: Macrolide-resistant Group A Streptococci, penicillin-resistant Group B Streptococci (note that CDC also has these organisms as Concerning)
- Tweaked: Penicillin-non-susceptible Streptococcus pneumoniae becomes Macrolide-R S. pneumoniae; MRSA (methicillin-resistant S. aureus) is retained but vancomycin-I/R is dropped.
- Re-arranged: A bit of shuffling has gone on at the genus level within the Enterobacterales (Enterobacteriaceae) but we retain the carbapenem-R and ESBL groups as Critical.
- Added as a standalone: Rifampin-resistant M. tuberculosis is now added at the Critical level rather than just being a shout-out as it was in 2017. This also aligns with CDC having included it as Serious in their PPLs.
These focused and carefully targeted updates all make good sense to me as an evolution in our common goals. The PPLs are fundamental to the targeting of the global R&D ecosystem and having a globally similar set of relative stable goals is necessary given the enormous amount of work (and time) required to bring forward new therapies (see the 9 April 2024 newsletter entitled “48,015 → 0: Antibacterial Discovery Is Hard. Really, Really Hard” if you need a refresh on this).
Well done to the WHO and its colleagues! Per the methods, this was an extensive review involving colleagues from around the world … many thanks to all for their time and effort!
All best wishes, –jr
John H. Rex, MD | Chief Medical Officer, F2G Ltd. | Operating Partner, Advent Life Sciences. Follow me on Twitter: @JohnRex_NewAbx. See past newsletters and subscribe for the future: https://amr.solutions/blog/. All opinions are my own.
- CARB-X has open calls that span four areas: (i) Therapeutics for Gram-Negatives, (ii) Prevention for Invasive Disease, (iii) Diagnostics for Neonatal Sepsis, and (iv) Proof-Of-Concept for Diagnosing Lower-Respiratory-Tract Infections. See this 6 Mar 2024 newsletter for a discussion of the call and go here for the CARB-X webpage on the call. There are multiple opportunities to submit — see the CARB-X webpage for details.
- BARDA’s long-running BAA (Broad Agency Announcement) for medical countermeasures (MCMs) for chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases is now BAA-23-100-SOL-00004 and offers support for both antibacterial and antifungal agents (as well as antivirals, antitoxins, diagnostics, and more). Note especially these Areas of Interest: Area 3.1 (MDR Bacteria and Biothreat Pathogens), Area 3.2 (MDR Fungal Infections), and Area 7.2 (Antibiotic Resistance Diagnostics for Priority Bacterial Pathogens). Although prior BAAs used a rolling cycle of 4 deadlines/year, the updated BAA released 26 Sep 2023 has a 5-year application period that ends 25 Sep 2028 and is open to applicants regardless of location: BARDA seeks the best science from anywhere in the world! See also this newsletter for further comments on the BAA and its areas of interest.
- HERA Invest was launched August 2023 with €100 million to support innovative EU-based SMEs in the early and late phases of clinical trials. Part of the InvestEU program supporting sustainable investment, innovation, and job creation in Europe, HERA Invest is open for application to companies developing medical countermeasures that address one of the following cross-border health threats: (i) Pathogens with pandemic or epidemic potential, (ii) Chemical, biological, radiological and nuclear (CBRN) threats originating from accidental or deliberate release, and (iii) Antimicrobial resistance (AMR). Non-dilutive venture loans covering up to 50% of investment costs are available. A closing date is not posted insofar as I can see — applications are accepted on a rolling basis; go here for more details.
- The AMR Action Fund is open on an ongoing basis to proposals for funding of Phase 2 / Phase 3 antibacterial therapeutics. Per its charter, the fund prioritizes investment in treatments that address a pathogen prioritized by the WHO, the CDC and/or other public health entities that: (i) are novel (e.g., absence of known cross-resistance, novel targets, new chemical classes, or new mechanisms of action); and/or (ii) have significant differentiated clinical utility (e.g., differentiated innovation that provides clinical value versus standard of care to prescribers and patients, such as safety/tolerability, oral formulation, different spectrum of activity); and (iii) reduce patient mortality. It is also expected that such agents would have the potential to strongly address the likely requirements for delinked Pull incentives such as the UK (NHS England) subscription pilot and the PASTEUR Act in the US. Submit queries to contact@amractionfund.com.
- INCATE (Incubator for Antibacterial Therapies in Europe) is an early-stage funding vehicle supporting innovation vs. drug-resistant bacterial infections. The fund provides advice, community, and non-dilutive funding (€10k in Stage I and up to €250k in Stage II) to support early-stage ventures in creating the evidence and building the team needed to get next-level funding. Details and contacts on their website (https://www.incate.net/).
- These things aren’t sources of funds but would help you develop funding applications
- AiCuris’ AiCubator offers incubator support to very early stage projects. Read more about it here.
- The Global AMR R&D Hub’s dynamic dashboard (link) summarizes the global clinical development pipeline, incentives for AMR R&D, and investors/investments in AMR R&D.
- Diagnostic developers would find valuable guidance in this 6-part series on in vitro diagnostic (IVD) development. Sponsored by CARB-X, C-CAMP, and FIND, it pulls together real-life insights into a succinct set of tutorials.
- In addition to the lists provided by the Global AMR R&D Hub, you might also be interested in my most current lists of R&D incentives (link) and priority pathogens (link).
John’s Top Recurring Meetings
Virtual meetings are easy to attend, but regular attendance at annual in-person events is the key to building your network and gaining deeper insight. My personal favorites for such in-person meetings are below. Of particular value for developers are the AMR Conference and the ASM-ESCMID conference. Hope to see you there!
- [REGISTRATION IS NOW OPEN] 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. Go here to register!
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. Save the date! More details to come!
- 25-26 February 2025 (Basel, Switzerland): The 9th AMR Conference 2025. Go here to register!
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. Go here for details.
Upcoming meetings of interest to the AMR community:
- 14 May 2024 (French Embassy, Washington, DC, in person, 3-7p ET): Jointly sponsored by bioMérieux and the French Embassy in the United States, an in-person event entitled “Rising to the Challenge: United against Antimicrobial Resistance.” This program will focus on AMR with a diagnostic twist. Go here to register. Deadline to register is 6 May and space is limited, so move quickly to signup if you are in the DC area on that day.
- 14 May 2024 (New York City, NY, in person and online, 6-8p ET): Jointly sponsored by FP and IFPMA, we have an event entitled “From Resistance to Resilience: Reinforcing the response to AMR.” If you are in NYC instead of DC, you can attend instead this event that IFPMA is organizing in partnership with Foreign Policy (FP) just before the UN AMR Multistakeholder Hearings. Go here to register; I don’t see a registration deadline but would assume you should move quickly!
- 15 May 2024 application deadline: There are 11 fully funded PhD training slots for MYCOS, a training program focused on research on antifungal resistance based on a One Health Approach. The program is based in Austria at the Medical University Innsbruck (MUI) and the University of Innsbruck (UIBK). Go here for details and to apply.
- 15 May 2024 (in person, New York City, USA; there will be a listen-only webstream): A multistakeholder Hearing for 2024 UNGA HLM on AMR will be held by the Quadripartite Joint Secretariat (QJS-AMR). This is part of the prep for the Sep 2024 High-Level Meeting (HLM) on AMR. Preregistration by 24 April 2024 is required — go here for the registration portal.
- 21-22 May 2024 (hybrid in-person and online, Falls Church, VA, 9a-4p ET both day): 25th PACCARB public meeting. The primary topic is a report to the Secretary of Health and Human Services. Additional topics will cover AMR in conflict zones, the environment, and the voice of the patient.
- As a special event during the meeting, the first of a pair of short films on AMR (18-19 minutes each) will be screened. Collectively titled HOLOBIOME, these films were created with funding from NovoNordisk Foundation and with advisory support from WHO. The films are from the same team (small-r.com) who created the excellent 2015 RESISTANCE movie and cover (i) the story of AMR in a young kidney transplant patient (ii) a sci-fi / documentary hybrid exploring the need for innovation.
- Go here to register for the PACCARB meeting; go here for a preview trailer (~2 minutes) of the short films.
- [NEW] 22 May 2024 (in person, Washington, DC, Capital Visitor’s Center, 5p ET): Additional screening of HOLOBIOME. See discussion just above of the PACCARB-related screening. Go here for details and to register.
- 26-31 May 2024 (Montreal, Canada): EDAR7, the McGill AMR Centre’s 7th edition of their Environmental Dimension of Antimicrobial Resistance conference. Go here for details; final abstract deadline is 21 Dec 2023.
- 28-29 May 2024 (in person, Uppsala, Sweden): Uppsala Antibiotic Days, a broad-ranging 2-day program hosted by the Uppsala Antibiotic Center. Go here for details and to register.
- 30-31 May 2024 (face-to-face in Rockville, Maryland as well as online, 8.30-5.30p ET on 30 May, 9-2.40p on 31 May): NIAID-sponsored workshop entitled “Towards realizing the promise of adjunctive immune therapy for invasive fungal infections”. The agenda covers host immunity to invasive fungal infections, immune modulators in the context of fungal infections; and strategies for testing immune modulators as adjunctive therapy. Go here for more details and to register.
- 9-13 June 2024 (in person, Ascona, Switzerland): “New Approaches to Combat Antibiotic-Resistant Bacteria, 2nd Edition” is a Sunday-Thursday residential workshop focused on the deep biology of AMR. Sponsored by NCCR AntiResist (a Swiss National Science Foundation consortium), the scientific program has the feel of a Gordon Conference. Space is limited, so you are encouraged to apply promptly — go here for details.
- 13-17 June 2024 (Atlanta, Georgia): ASM Microbe, the annual meeting of the American Society for Microbiology. You can’t register yet, but you can go here for general details.
- [NEW] 27 June 2024 (virtual, 5p-630p CEST): GARDP REVIVE Webinar “Progressing an antibacterial drug discovery project – an SME perspective”. Click here for details.
- 17-20 Sep 2024 (Porto, Portugal): ASM/ESCMID Joint Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. See Recurring Meetings list, above.
- 16-20 Oct 2024 (Los Angeles, USA): IDWeek 2024, the annual meeting of the Infectious Diseases Society of America. See Recurring Meetings list, above.
- 19-27 Oct 2024 (Annecy, France, residential in-person program): ICARe (Interdisciplinary Course on Antibiotics and Resistance). Now in its 8th year, Patrice Courvalin directs the program with the support of an all-star scientific committee and faculty. The resulting soup-to-nuts training covers all aspects of antimicrobials, is very intense, and routinely gets rave reviews! Seating is limited, so mark your calendars now if you are interested. Applications open in March 2024 — go here for more details.
- 4-5 Dec 2024 (in person, Washington, DC): “Fungal Dx 2024: Fungal Diagnostics in Clinical Practice” is a 2-day in-person workshop organized by ISHAM‘s Fungal Diagnostics Working Group. The program and registration links are available at https://fungaldx.com/; the agenda is comprehensive and features an all-star global list of speakers.
- 11-15 April 2025 (Vienna, Austria): ESCMID Global 2025, the annual meeting of the European Society for Clinical Microbiology and Infectious Diseases. See Recurring Meetings list, above.